Solutions for
Cerebrovascular
Disease
Lamiflo™ is the first non-pharmacologic for cerebrovascular disease.
Lamiflo™ is a new approach to an old problem

Lamiflo™ is a disruptive technology in current stroke protocol because it can be administered in the pre-hospital phase; will increase the number of patients qualifying for treatment by thrombolysis or intra-arterial thrombectomy; can be used in patients who do not qualify for either treatment; and could be used in patients, days to months after a stroke, who are still at risk for a second stroke.
A global
emergency
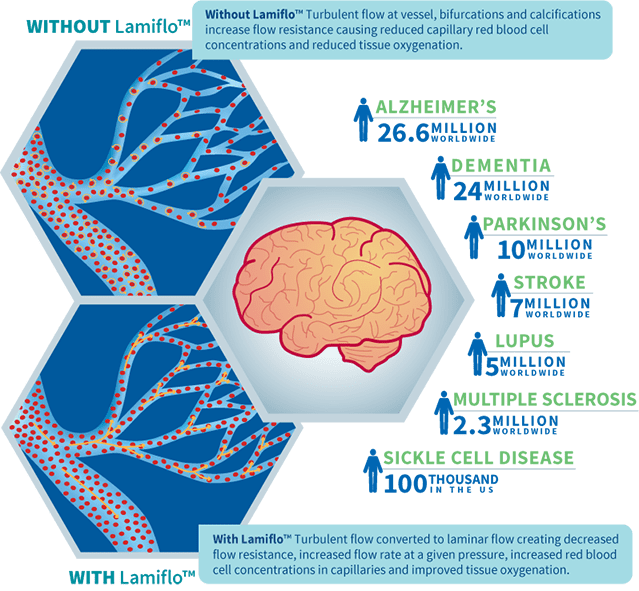
Cerebrovascular disease is a global emergency as shown in the infographic on the right. Lamiflo™ is considered disruptive technology in its application to acute large vessel occlusion (LVO) stroke as in our current focus because it can be administered in the pre-hospital phase; will increase the number of patients qualifying for treatment by thrombolysis or intra-arterial thrombectomy; can be used in patients who do not qualify for either; and could be used in patients, days to months after a stroke, who are still at risk for a second stroke.
Our goals
- Apply for a proof of concept NIH Phase I SBIR grant for Lamiflo™.
- Apply for NIH Phase II SBIR grant for IND enabling studies and Phase I clinical safety study on healthy volunteers and commercialization plan.
- Obtain the IND and conduct Phase I clinical safety study in healthy volunteers.
- With Partner support perform a Multicenter Phase II Clinical Safety and Efficacy Trial of Lamiflo™ in acute stroke patients with large vessel occlusion before or after Thrombolysis (TLY) or Intra-Arterial Thrombectomy (IAT).
- Perform a Phase III Clinical Trial to compare the effectiveness of Lamiflo™ to other therapeutic procedures available again in LVO acute stroke patients; and
- Obtain FDA approval for the medication.
- Perform a Phase IV clinical trial in LVO acute stroke patients to obtain long term safety and other benefits of Lamiflo™ treatment.
- Commercialization of Lamiflo™.
Interested in knowing more?
Would you like to learn more about Shearit?
Please feel free to contact us by filling in the information below.
